Indication for use:
Restoration and maintenance of normal gut microbiota in cases of:
- antibiotic use, chemotherapy, hormone therapy, etc.
- diarrhea caused by viral or bacterial infections of the gastrointestinal tract
- functional bowel disorders (including IBS, functional: constipation, diarrhea, bloating, or abdominal pain)
- inflammatory bowel diseases (IBD, including non-specific ulcerative colitis and Crohn’s disease)
- chronic constipation in elderly patients
- dietary errors, climate change, water consumption, and diet changes
- fermentopathies (lactose intolerance, gluten intolerance, etc.)
- anti-H. pylori therapy
Improves the absorption processes of calcium, iron, and vitamins in malabsorption syndrome.
Supports immune system health.
Directions for use:
One sachet once a day in the morning or evening during meals.
The contents can be mixed with food, water, milk or yogurt.
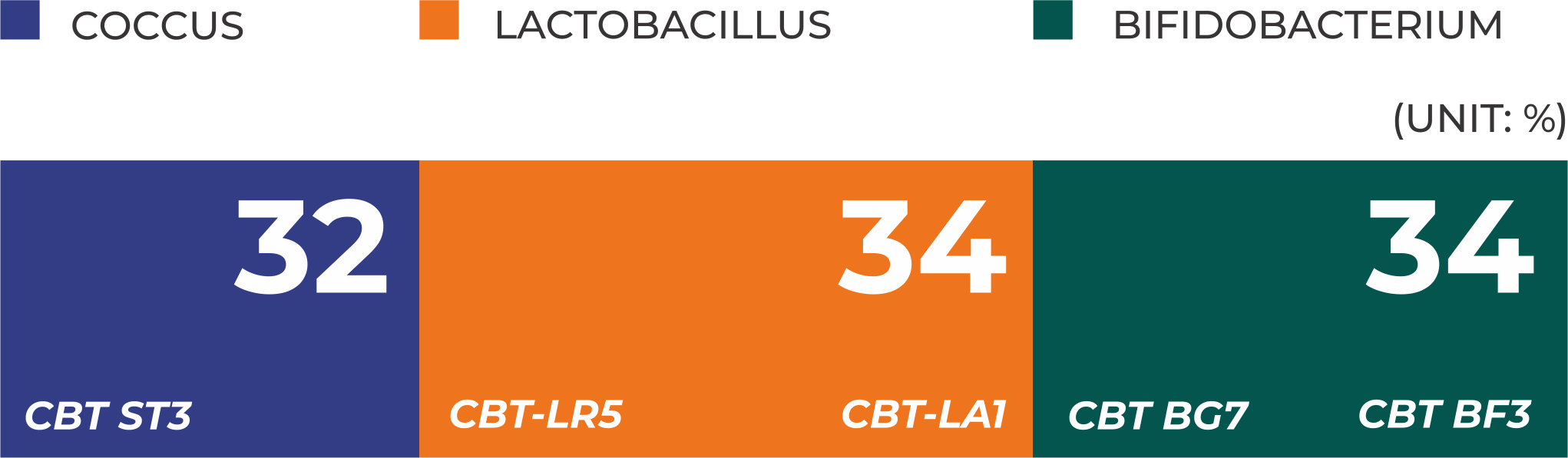
Exclusive strains from Cell Biotech (CBT), South Korea:
- HUMAN-DERIVED STRAINS. Strains derived from healthy humans are more likely to be compatible with the human body, leading to better colonization and survival in the gut environment compared to strains from other sources.
- Genetically identified through 16S rRNA sequencing.
- Proven health safety via antibiotic resistance tests and non-toxicity, and GRAS notice recognized by U.S. FDA
- Strains are deposited and registered in certified microorganism banks: Korean Collection for Type Cultures (KCTC), German Collection of Microorganisms and Cell Cultures (DSMZ), National Center for Biotechnology Information (NCBI).
GLOBAL QUALITY CONTROL STANDARDS

100 TIMES HIGHER SURVIVAL RATE
More than 90% of good bacteria are orally consumed but they are killed by gastric acid and bile salt. Our superior coating technology ensures a probiotic survival rate in the intestine that is 100 times higher than that of uncoated probiotics.
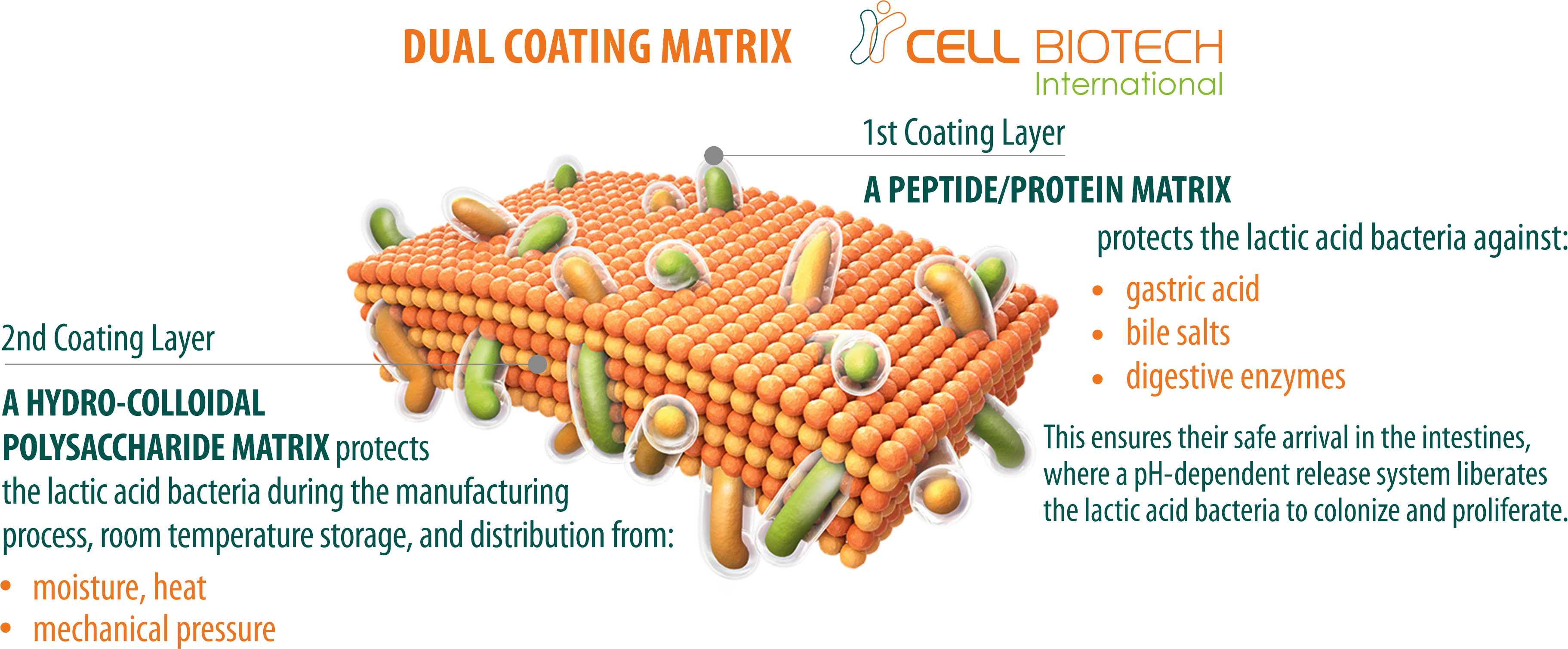
WORLDWIDE PATENTS
CellBiotech’s LAP2PRO double-layer coating technology is patented worldwide, including in Korea, the USA, Europe, China, and Japan. These global patents enable us to export to 53 countries worldwide.

Scientifically validated strain ratio
takes into account the characteristics of each strain and the intestinal environment to achieve synergy and optimal results.
PUBLICATIONS AND STUDIES:
| Journal of Gastroenterology:
The intake of a double-coated probiotic (2.5 × 109 CFU, Streptococcus thermophilus, Lactobacillus acidophilus, Lactobacillus rhamnosus, Bifidobacterium longum, Bifidobacterium bifidum, and fructooligosaccharide) significantly reduces gastrointestinal discomfort, promotes stool normalization, and has a positive effect on the treatment of diarrhea-predominant irritable bowel syndrome (IBS) compared to uncoated probiotics.
Journal of Gastroenterology 52(4) • May 2016 |
| Journal of clinical biochemistry and nutrition:
A four-week administration of a probiotic mixture (Streptococcus thermophilus, Lactobacillus acidophilus, Lactobacillus rhamnosus, Bifidobacterium longum, Bifidobacterium bifidum) led to an increase in probiotic concentration in feces and improvement in diarrhea symptoms in patients with irritable bowel syndrome (IBS). Changes in the total symptom score, which includes 10 IBS symptoms, and in the subcategories (pain, constipation, diarrhea, and bloating/gas) were evaluated. The proportion of patients with adequate symptom relief was higher in the probiotic group compared to the placebo group.
Journal of clinical biochemistry and nutrition 57(2): 129–134. • September 2015 |
| Journal of Gastroenterology and Hepatology:
The study evaluated the effectiveness of a four-week treatment with multispecies probiotics (B. longum, B. bifidum, B. lactis, L. acidophilus, L. rhamnosus, Strep. Thermophilus) for irritable bowel syndrome (IBS) and changes in gut microbiota. Significant symptom relief was observed in 68% of patients in the probiotic group compared to 37.5% in the placebo group (P < 0.05). The probiotic group also showed improvements in abdominal pain/discomfort and bloating. Microflora analysis revealed a significant increase in B. lactis, L. rhamnosus, and S. thermophilus in the probiotic group.
Effect of multispecies probiotics on irritable bowel syndrome: a randomized, double-blind, placebo-controlled trial. Jun Sik Yoon, Won Sohn, Oh Young Lee, Sang Pyo Lee, Kang Nyeong Lee, Dae Won Jun, Hang Lak Lee, Byung Chul Yoon, Ho Soon Choi, Won-Seok Chung, Jae-Gu Seo |
| Europian Journal of Pediatrics:
The use of multispecies synbiotics (2.5 × 109 CFU, Streptococcus thermophilus, Lactobacillus acidophilus, Lactobacillus rhamnosus, Bifidobacterium longum, Bifidobacterium bifidum, and fructooligosaccharide) reduces the duration of acute diarrhea by 36 hours and shortens hospital stay by approximately 1 day in children with acute diarrhea.
The effect of a multispecies synbiotic mixture on the duration of diarrhea and length of hospital stay in children with acute diarrhea in Turkey: Single blinded randomized study.
|
| Archives of Pharmacal Research
The use of double-coated multispecies probiotics in elderly individuals with functional constipation leads to an improvement in symptoms: straining during defecation, the sensation of blockage in the rectum during attempts to defecate, and frequency of defecation.
Yeun, Y., Lee, J. Arch. Pharm. Res. 38, 1345–1350 • July 2015 |
| International Medical Journal Acta Medica Mediterranea
The addition of a synbiotic (S. Thermophilus, B. longum, B. bifidum, L. acidophilus, L. rhamnosus) to standard triple therapy improves H. pylori eradication rates and reduces the frequency of antibiotic-related side effects. In the synbiotic group, the success rate of treatment was 88.4%, compared to 68.8% in the control group (p<0.05).
Synbiotic therapy increases eradication rate in Helicobacter pylori eradication. Önder Şahin, Atakan Yeşil, Ebubekir Şenateş, Mehmet Fatih Akdoğan, Sevki Konür, Emrullah Erdem, Mehmet Sinan dal, Tarkan Karakan. Acta Medica Mediterranea, 29(3):569-573 • January 2013 |
| International scientific journal Beneficial Microbes
The study showed that a one-month course of NBL Probiotic GOLD synbiotic in children with primary obesity, in addition to standard treatment methods (reducing caloric intake and increasing physical activity), significantly contributes to the reduction of body weight and body mass index, as well as to the improvement of lipid profile and overall oxidative stress levels. Children who received the synbiotic demonstrated better results in weight reduction, improvement of lipid profile, and reduction of oxidative stress levels compared to the group that received only standard treatment. For the first time, it was demonstrated that synbiotics have a positive effect on oxidative stress in children with obesity, while also contributing to weight loss.
Effects of synbiotic on anthropometry, lipid profile and oxidative stress in obese children. N. Ipar, S. Durmus Aydogdu, G. Kilic Yildirim, M. Inal, I. Gies, Y. Vandenplas, E.C. Dinleyici Beneficial Microbes, 6(6):775-782 • August 2015 |
| International Scientific Journal Scientific Reports
A study showed that a 12-week administration of the GOLD probiotic in obese patients with nonalcoholic fatty liver disease (NAFLD) led to a significant reduction in body mass index (BMI) by −0.48 kg/m² (p = 0.0024), intrahepatic fat (IHF) from 16.3% to 14.1% (p = 0.032), and triglyceride levels by -34.0 mg/dL (p = 0.0033). These results confirm the positive effect of probiotics on body weight and metabolic parameters in this patient group.
Sang Bong Ahn, Dae Won Jun, Bo-Kyeong Kang, Jong Hyun Lim, Sanghyun Lim, Myung-Jun Chung Scientific Reports, 9:5688 • April 2019 |
Contraindications:
Individual intolerance to the product components.
Shelf life:
2 years
Storage conditions:
Does not require refrigeration.
Store in a place inaccessible to children at a temperature not exceeding 25°C.














