Indication for use:
Restoration and maintenance of normal gut microbiota in cases of:
- antibiotic use, chemotherapy, hormone therapy, etc.
- diarrhea caused by viral or bacterial infections of the gastrointestinal tract, including rotavirus infection
- enzymopathies (lactose deficiency, gluten intolerance, etc.)
Support for the formation and functioning of the immune system.
Prevention of allergic and atopic diseases (food allergies, atopic dermatitis, allergic rhinitis, bronchial asthma).
Directions for use:
One sachet once or twice a day, in the morning or evening with food.
The contents can be mixed with food, water, milk or yogurt.
Exclusive strains from Cell Biotech (CBT), South Korea:
- HUMAN-DERIVED STRAINS. Strains derived from healthy humans are more likely to be compatible with the human body, leading to better colonization and survival in the gut environment compared to strains from other sources.
- Genetically identified through 16S rRNA sequencing.
- Proven health safety via antibiotic resistance tests and non-toxicity, and GRAS notice recognized by U.S. FDA
- Strains are deposited and registered in certified microorganism banks: Korean Collection for Type Cultures (KCTC), German Collection of Microorganisms and Cell Cultures (DSMZ), National Center for Biotechnology Information (NCBI).
GLOBAL QUALITY CONTROL STANDARDS

100 TIMES HIGHER SURVIVAL RATE
More than 90% of good bacteria are orally consumed but they are killed by gastric acid and bile salt. Our superior coating technology ensures a probiotic survival rate in the intestine that is 100 times higher than that of uncoated probiotics.
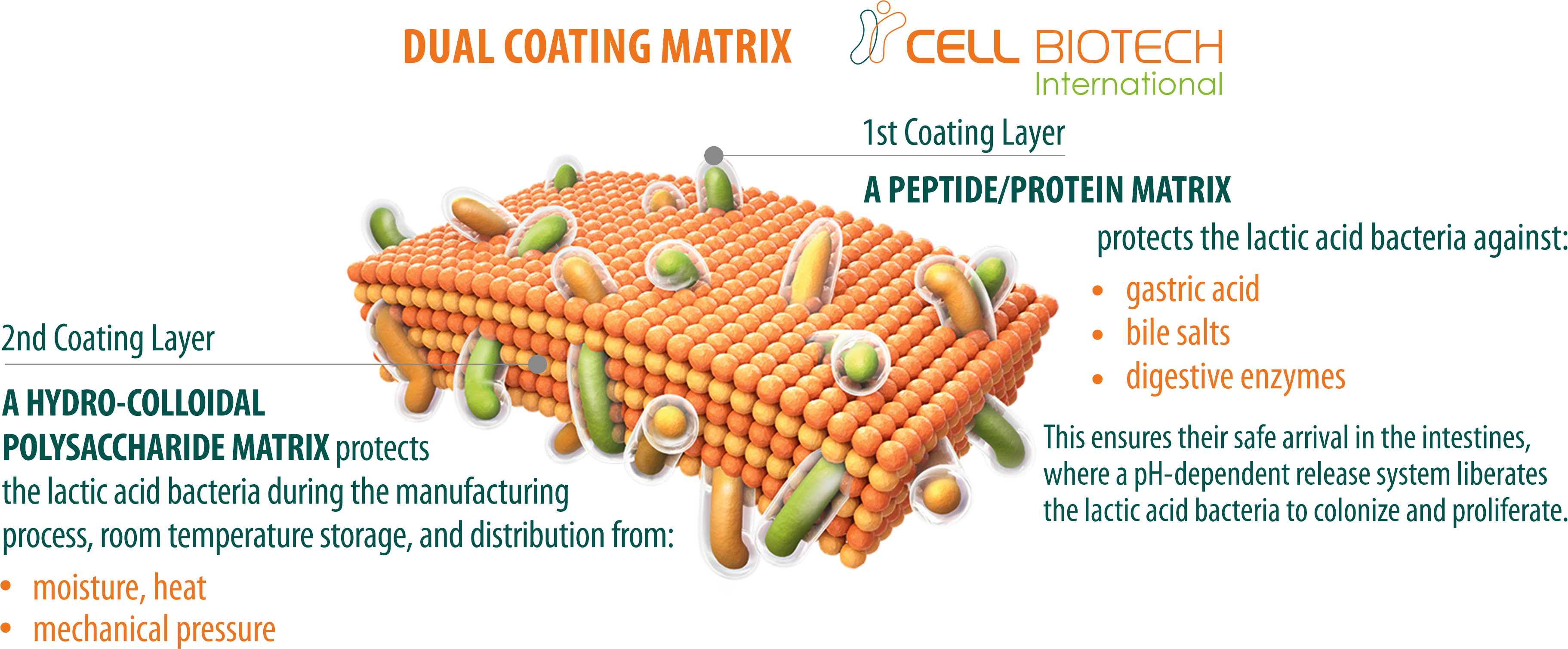
WORLDWIDE PATENTS
CellBiotech’s LAP2PRO double-layer coating technology is patented worldwide, including in Korea, the USA, Europe, China, and Japan. These global patents enable us to export to 53 countries worldwide.

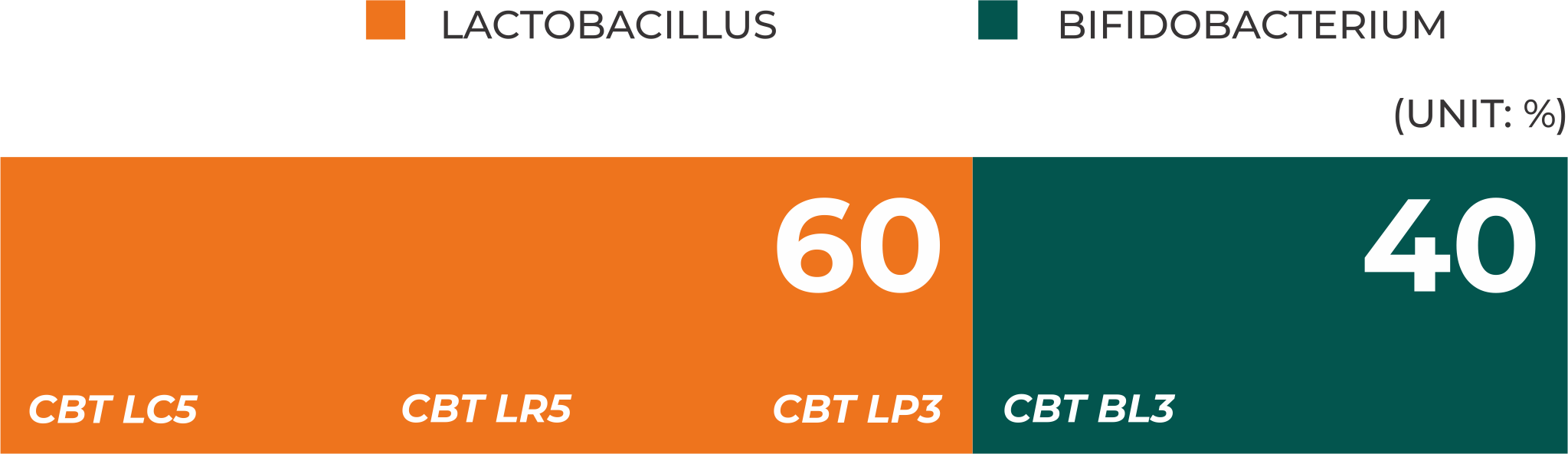
Scientifically validated strain ratio
takes into account the characteristics of each strain and the intestinal environment to achieve synergy and optimal results.
PUBLICATIONS AND STUDIES:
| European Journal of Pediatrics:
The use of a multi-strain synbiotic (Lactobacillus casei, Bifidobacterium lactis, Lactobacillus rhamnosus, Lactobacillus plantarum, fructooligosaccharide, galactooligosaccharide) helps reduce skin manifestations of atopic dermatitis in children.
Ibáñez MD, Rodríguez Del Río P, González-Segura Alsina D, Villegas Iglesias V. Eur J Pediatr. 177(12):1851-1858 • December 2018 |
| Toxicological Research:
The multi-strain synbiotic (Lactobacillus casei, Bifidobacterium lactis, Lactobacillus rhamnosus, Lactobacillus plantarum, fructooligosaccharide, galactooligosaccharide) with dual coating inhibits the development of skin lesions in atopic dermatitis and serves as an essential complement to primary therapy.
A Probiotic Preparation Alleviates Atopic Dermatitis-Like Skin Lesions in Murine Models. Min-Soo Kim, Yeo-Sang Yoon, Jin-Eung Kim, Jae-Gu Seo Toxicological Research 32(2):149-158 • April 2016 |
| Frontiers in Microbiology:
In this study, the ATP probiotic (Lactobacillus casei, Bifidobacterium lactis, Lactobacillus rhamnosus, Lactobacillus plantarum) demonstrated the ability to reduce IgE levels by decreasing Th2 activity and enhancing Th1 response, thereby preventing skin inflammation and hyperplasia. The ATP probiotic shows significant preventive potential in the treatment of atopic dermatitis symptoms and may become a promising immunomodulatory agent for patients with this condition.
A probiotic mixture regulates T cell balance and reduces atopic dermatitis symptoms in mice. Han Wool Kim, Rira Hong, Eun Young Choi, KeeSun Yu, Narae Kim, Jin Yi Hyeon, Kwang Keun Cho, In Soon Choi, Cheol-Heui Yun Frontiers in Microbiology 9:2414. 10.3389/fmicb.2018.02414 • October 2018 |
Contraindications:
Individual intolerance to the product components.
Shelf life:
2 years
Storage conditions:
Does not require refrigeration.
Store in a place inaccessible to children at a temperature not exceeding 25°C.














